HOW FASTER USFDA NODS WILL HELP INDIAN GENERIC DRUG MAKERS
MUMBAI: A long spell of sluggish approvals for generic drugs in the US may be nearing an end with early signs of a rebound. A backlog of as many as 4,000 pending applications at the US Food and Drug Administration (USFDA), of which a fourth is estimated to have been filed by Indian firms, is awaiting clearance. Fortunes of generic drug makers to a great extent hinges on their approval timelines. Experts say going by the current run rate, the FDA may clear at least 470 such products this year,a sharp uptick from 409 approvals in 2014.
The number may even cross 500 if the FDA does not seek additional details via complete response letters. Some of these products may mark the entry of new companies in areas that have so far seen limited competition, implying a cut in profit margins. As part of a fixed five-year plan, the Generic Drug User Fee Amendments were initiated in 2012 in the US to facilitate a speedy approval process, with industry players paying a fee to supplement costs of reviewing the applications and inspecting facilities.
While the first two years of the plan did not show a perceptible change in terms of faster approvals, the FDA had shared details on industry forums about additional hiring for review processes and investments made to accelerate the pace of approvals. Some of the Indian drug makers have already seen faster approvals this year. A recent report by HSBC analyst Girish Bakhru named Aurobindo Pharma as a company that has witnessed a higher number of approvals.
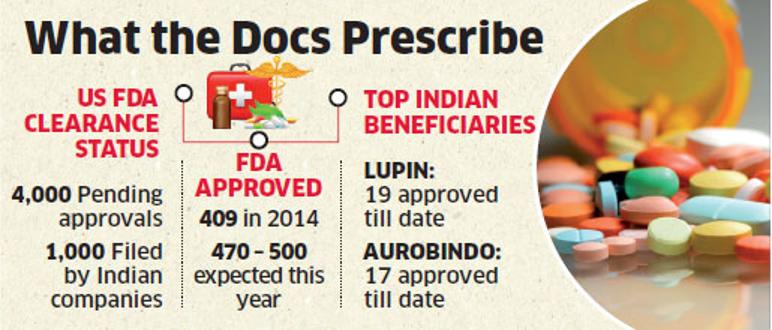
It indicated the company has a better approvals outlook and can be expected to see growing sales of its injectables towards the end of 2015-16. Aurobindo has gained 17 FDA approvals so far this year, second in the chart among Indian firms.
Lupin has emerged as the biggest beneficiary of the GDUFA process as the company gained approvals for 19 products in 2015. Last week, the company disclosed of an approval, its third in five days, for a delayed release version of gastric ulcer and heartburn drug omeprazole.
A few big companies, however, are lagging in terms of approvals like Dr. Reddy's, Sun and Cadila. Applications from these companies are mostly held back due to their internal manufacturing related issues rather than the FDA's application review process.
Over the last 12 months, Sun has undertaken a corrective action plan at its crucial site at Halol in Gujarat.Dr. Reddy's, similarly, is seeing a delay in approvals of big-ticket drugs owing to observations cited by the FDA at its Srikakulam site in Andhra Pradesh. Cadila's Moraiya facility in Gujarat is also expected to gain clearance for over a dozen products, once those pass through the FDA's checks. Analysts also note that the volume of filings for generic products by Indian companies surged, adding to the existing logjam.
Source:
http://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/how-faster-usfda-nods-will-help-indian-generic-drug-makers/articleshow/48751878.cms




